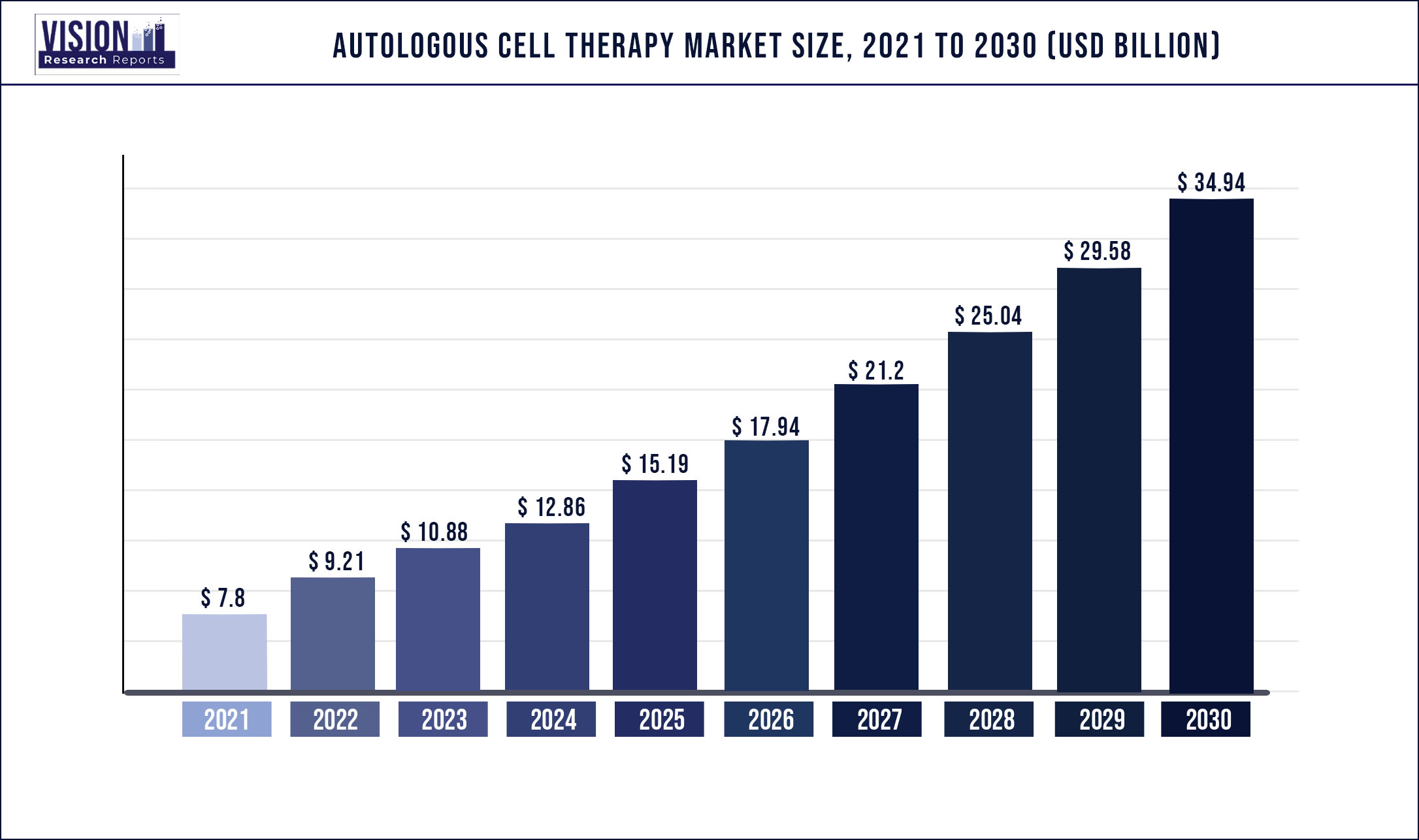
The global autologous cell therapy market was valued at USD 7.8 billion in 2021 and it is predicted to surpass around USD 34.94 billion by 2030 with a CAGR of 18.13% from 2022 to 2030.
Autologous Cell Therapy (ACT) is a novel therapeutic intervention that uses an individual’s cells for in-vitro culture and re-introduction into the body. The global ACT market is set out for major growth due to the rise in the number of people suffering from conditions like neurodegenerative disorders, autoimmune disorders, multiple sclerosis, cancer, and so on. These therapies are also used for niche therapeutic utility such as orthopedics and wound healing. Moreover, the rise in the proportion of the geriatric population across the globe coupled with the demand for regenerative medicine, are positively impacting the market growth.
The therapy is intended to replace unhealthy cells with healthy ones mostly by induced expression or the removal of disease causing or dysfunctional cells using immune cells. Advancements in disease biology, coupled with trending technologies such as cell culture, genetic, and protein engineering are conducive to growth of this market. For instance, in December 2021, researchers at the Columbia University Irving Medical Center found out that gene-based cell therapy for SCD restores blood cells to their normal shape and eliminates clinical complications for at least three years.
ACT offer numerous immunological advantages such as eliminating the possibility of graft-versus-host disease (GvHD), which can cause inflammatory symptoms such as enteritis, and dermatitis. They are preferred to achieve Immuno-compatibility for transplantation and also to rule out the need for immunosuppression. Utility of somatic cells for reprogramming into iPSCs to combat Parkinson’s disease. Many studies are underway for using cell therapy to treat Parkinson’s disease. For instance, in May 2022, Aspen Neuroscience plans to advance its lead candidate for Parkinson’s disease and raised USD 147.5 million in Series B financing.
This therapy is being extensively used for devising novel cancer therapeutics and yielded promising outcomes in many studies carried out by researchers across the globe to curb the proliferation of tumorous growth. For instance, in May 2022, Autolus plc presented its research findings at the American Society of Gene & Cell Therapy (ASGCT) about CAR-T cell therapy to treat cancer by using a versatile constitutive cytokine receptor (CCR) system to leverage T cell therapies and to avoid systemic toxicity. Similarly, in April 2022, Autolus Therapeutics plc declares that the U.S. FDA grants an RMAT (Regenerative-Medicine Advanced Therapy) designation for its CAR-T cell therapy called obecabatagene autoleucel (intended for use in the treatment of acute lymphocytic leukemia) that is now undergoing Phase-II trial.
Advantages include the minimization of risks from bio-incompatibility, and disease transmission risks associated with grafts and are therefore used to bioengineer skin substitutes, aid wound healing, reversal of chronic inflammation, treat ulcers, and leverage postoperative healing. There are many corporate developments in terms of acquisitions and expansions to effectively market wound healing technologies. For instance, in June 2022, Switzerland based company, Healiva acquired two innovative cell therapy assets, EpiDex and Healiva002 from Smith+Nephew so as to establish a broad spectrum of personalized wound care by comprehensive use of enzyme technology, ACT, and associative medical devices.
However, hassles in harvest processes, the logistic intricacies (involving clinical procedures) are hindrances to the growth of the market. It is crucial that the procedures are well-timed and coordinated to mitigate the risk of cross-contamination. Turnaround time of several weeks can make the option not favorable in cases where clinicians are making a last attempt to save the life of a patient.
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 7.8 billion |
| Revenue Forecast by 2030 | USD 34.94 billion |
| Growth rate from 2022 to 2030 | CAGR of 18.13% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Source, Application, End-Use, Region |
| Companies Covered |
BrainStorm Cell Therapeutics, Holostem Terapie Avanzate S.R.L, Lineage Cell Therapeutics Inc., Pharmicell Co. Inc, Opexa Therapeutics, Caladrius Biosciences Inc, Castle Creek Biosciences Inc, Regeneus Ltd., Opexa Therapeutics Inc, Takeda Pharmaceutical Company Limited, U.S. Stem Cell Inc, and Vericel Corporation |
Key Players
Market Segmentation
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Autologous Cell Therapy Market
5.1. COVID-19 Landscape: Autologous Cell Therapy Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Autologous Cell Therapy Market, By Source
8.1. Autologous Cell Therapy Market, by Source, 2022-2030
8.1.1 Bone Marrow
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Epidermis
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Mesenchymal stem cells
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Haematopoietic stem cells
8.1.4.1. Market Revenue and Forecast (2017-2030)
8.1.5. Chondrocytes
8.1.5.1. Market Revenue and Forecast (2017-2030)
8.1.6. Others
8.1.6.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Autologous Cell Therapy Market, By Application
9.1. Autologous Cell Therapy Market, by Application, 2022-2030
9.1.1. Cancer
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Neurodegenerative disorders
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Cardiovascular disorders
9.1.3.1. Market Revenue and Forecast (2017-2030)
9.1.4. Autoimmune disorders
9.1.4.1. Market Revenue and Forecast (2017-2030)
9.1.5. Orthopaedics
9.1.5.1. Market Revenue and Forecast (2017-2030)
9.1.5. Wound healing
9.1.5.1. Market Revenue and Forecast (2017-2030)
9.1.5. Others
9.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Autologous Cell Therapy Market, By End-Use
10.1. Autologous Cell Therapy Market, by End-Use, 2022-2030
10.1.1. Hospitals & Clinics
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Academics & Research
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Others
10.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Autologous Cell Therapy Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Source (2017-2030)
11.1.2. Market Revenue and Forecast, by Application (2017-2030)
11.1.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Source (2017-2030)
11.1.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.1.4.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Source (2017-2030)
11.1.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.1.5.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Source (2017-2030)
11.2.2. Market Revenue and Forecast, by Application (2017-2030)
11.2.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Source (2017-2030)
11.2.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.2.4.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Source (2017-2030)
11.2.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.2.5.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Source (2017-2030)
11.2.6.2. Market Revenue and Forecast, by Application (2017-2030)
11.2.6.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Source (2017-2030)
11.2.7.2. Market Revenue and Forecast, by Application (2017-2030)
11.2.7.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Source (2017-2030)
11.3.2. Market Revenue and Forecast, by Application (2017-2030)
11.3.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Source (2017-2030)
11.3.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.3.4.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Source (2017-2030)
11.3.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.3.5.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Source (2017-2030)
11.3.6.2. Market Revenue and Forecast, by Application (2017-2030)
11.3.6.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Source (2017-2030)
11.3.7.2. Market Revenue and Forecast, by Application (2017-2030)
11.3.7.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Source (2017-2030)
11.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.4.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Source (2017-2030)
11.4.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.4.4.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Source (2017-2030)
11.4.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.4.5.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Source (2017-2030)
11.4.6.2. Market Revenue and Forecast, by Application (2017-2030)
11.4.6.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Source (2017-2030)
11.4.7.2. Market Revenue and Forecast, by Application (2017-2030)
11.4.7.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Source (2017-2030)
11.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.5.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Source (2017-2030)
11.5.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.5.4.3. Market Revenue and Forecast, by End-Use (2017-2030)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Source (2017-2030)
11.5.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.5.5.3. Market Revenue and Forecast, by End-Use (2017-2030)
Chapter 12. Company Profiles
12.1. BrainStorm Cell Therapeutics
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Holostem Terapie Avanzate S.R.L
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Lineage Cell Therapeutics Inc.
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Pharmicell Co. Inc
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Opexa Therapeutics
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Caladrius Biosciences Inc
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Castle Creek Biosciences Inc
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Regeneus Ltd.
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Opexa Therapeutics Inc
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. Takeda Pharmaceutical Company Limited
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others