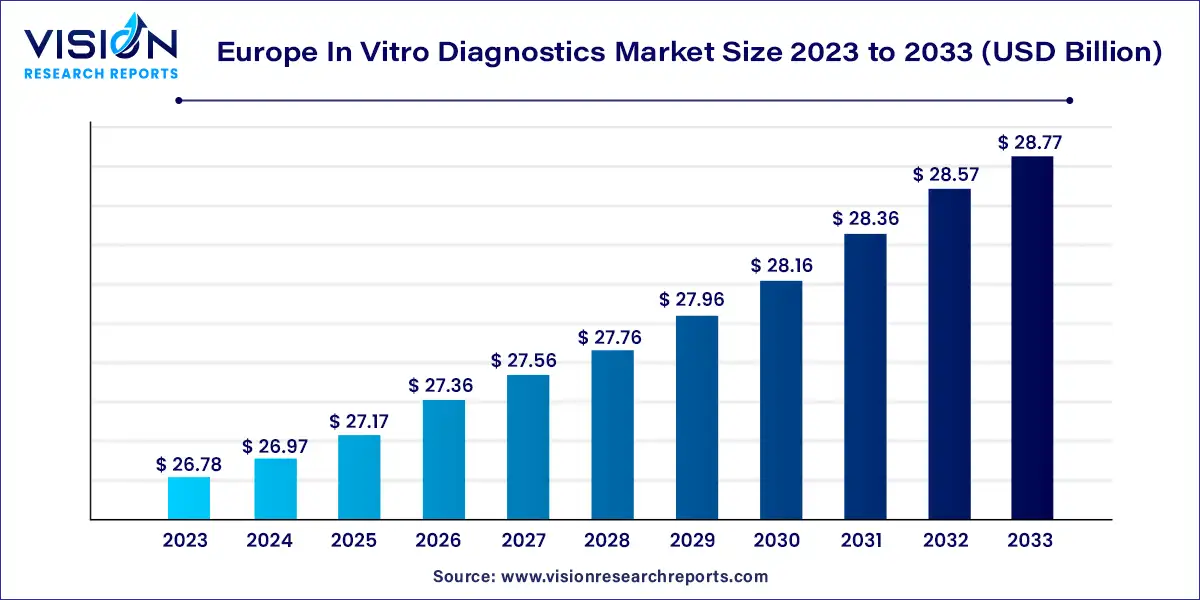
The Europe in vitro diagnostics market size was estimated at USD 26.78 billion in 2023 and it is expected to surpass around USD 28.77 billion by 2033, poised to grow at a CAGR of 0.72% from 2024 to 2033. The in vitro diagnostics (IVD) market in Europe stands as a crucial pillar within the broader healthcare landscape, facilitating accurate and timely disease diagnosis, prognosis, and therapeutic monitoring. This sector is characterized by dynamic advancements in technology, a rising prevalence of chronic diseases, and a concerted effort to enhance healthcare infrastructure.
The growth of the Europe in vitro diagnostics (IVD) market is propelled by an advancements in diagnostic technologies, such as molecular diagnostics and immunoassays, contribute to heightened accuracy and efficiency in disease detection. Secondly, the increasing prevalence of chronic diseases, including cardiovascular disorders and diabetes, accentuates the demand for reliable diagnostic solutions, fostering market expansion. Additionally, the aging demographic in Europe further amplifies the need for precise diagnostic tools to address a higher incidence of age-related diseases. Supportive government policies and initiatives aimed at enhancing healthcare infrastructure and research and development also play a pivotal role in driving market growth. Overall, the convergence of technological innovation, rising disease prevalence, demographic shifts, and governmental support collectively underpin the robust growth of the in vitro diagnostics market in Europe.
| Report Coverage | Details |
| Market Size in 2023 | USD 26.78 billion |
| Revenue Forecast by 2033 | USD 28.77 billion |
| Growth rate from 2024 to 2033 | CAGR of 0.72% |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
On the basis of product, the market is segmented into instruments, reagents, and software. The reagents segment dominated the market with a revenue share of around 66% in 2023, owing to a spike in the utilization of POC tests, self-testing kits, and various other cutting-edge items in in-vitro diagnosis. The rising volume of research and development efforts for detecting acute diseases is one of the primary reasons fueling the need for reagents and consumables. Furthermore, the growing priority placed on timely diagnosis in established and developing nations is increasing the proportion of patients getting routine tests, adding to the high growth of the category.
The instruments segment held the second largest share in 2023. The advancement in instruments provided a great avenue for the segment's growth. For instance, in September 2022, Sysmex Corporation launched the UD-1500 Completely Automated Urine Particle Analyzer for urine sediment evaluation. The product inherits the excellent capability and ease of use of the UF-5000, an entirely automated urine particle analyzer. The most popular IVD tools include cell imaging and analysis systems, slide processing systems, urine test strips, pregnancy tests, blood sugar monitoring systems, coagulation test systems, and PCR testing platforms.
On the basis of technology, the market is segmented into immunoassay, hematology, clinical chemistry, molecular diagnostics, coagulation, microbiology, and others. The immunoassay segment held the largest revenue share of over 36% in 2023, as immunoassays are being utilized significantly in alcohol and drug testing as well as cancer. Immunoassays are being furnished with modern technology to aid medical practitioners in generating timely diagnosis, boosting their acceptance.
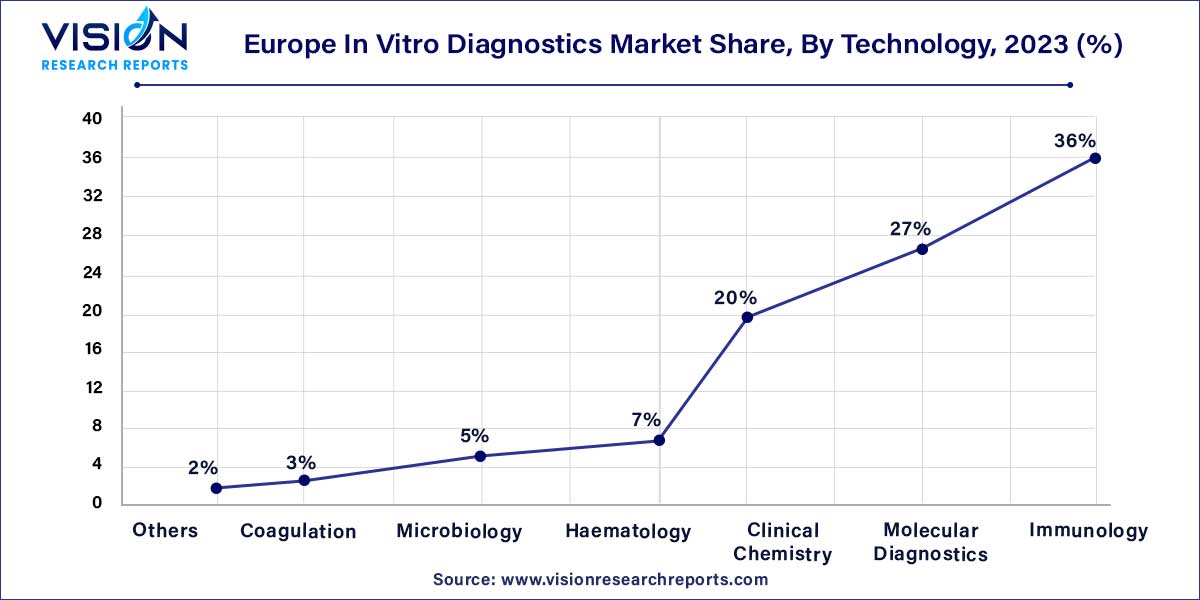
The microbiology and clinical chemistry segments are estimated to register the fastest CAGR of 4.45% over the forecast period of 2024 to 2033. A proportion of clinical chemistry is accomplished by utilizing high throughput apparatus with cutting-edge automation. However, point-of-care diagnostics continues to grow to address the need for quick diagnosis for chronic and contagious diseases. This factor is a crucial component influencing growth in clinical chemistry. Ongoing advancements in microbiology due to advancements in biotechnological research aimed at investigating the role of microbes in disease pathogenesis are also crucial factors driving the growth.
On the basis of application, the market is segmented into infectious diseases, diabetes, oncology/cancer, cardiology, nephrology, autoimmune diseases, drug testing, and others. The IVD-based infectious disease diagnostics market accounted for the largest market share owing to the rising prevalence of hospital-acquired infections and the high unmet medical needs pertaining to the effective diagnosis of infectious diseases. The substantial share of the market in this category is mainly related to the high frequency of infectious illnesses, the increasing number of elderly people, a growing need for early detection of diseases, and the government's attempt to increase the affordability of services geared towards infectious illness testing. In vitro diagnostics (IVD) is a widely prevalent diagnostic tool that complies with the minimum requirements. IVD kits have become popular and successful in diagnosing infectious illnesses.
The oncology segment is one of the fastest-growing segments due to the introduction of technologically advanced devices and the rising demand for companion diagnostics. The growing need for early disease identification and prevention stimulates development, improving diagnostic capabilities, and personalized medicine strategies, particularly in cancer diagnosis.
The European in vitro diagnostics market is segmented by end use into hospitals, laboratories, home care, and others. The hospital category dominated the overall segment, considering diagnostics are seamlessly integrated with other disciplines and comprehensive healthcare services are available. They have the facilities, resources, and qualified staff to run a successful IVD operation. Hospitals serve as primary places of care, primary points of referral, and providers of a wide range of medical services. Their range of clinical specialties guarantees precise diagnosis and analysis of IVD test findings. Furthermore, hospitals receive favorable funding and reimbursement policies and must follow strict regulatory guidelines to guarantee patient safety and test accuracy.
The home care segment is expected to grow at the fastest CAGR during forecast period due to the rising demand for rapid diagnostic services for patients at the time of care, the rising government initiatives aimed at lowering hospital stays to curb healthcare expenditure, and the increase in collaboration between the market players. Multiple partnerships between healthcare organizations and various MedTech firms offer a fantastic chance for the efficient implementation of healthcare services at the residence. For instance, Medtronic's Integrated Health Solutions (IHS) presently has 170 in-progress long-term relationships in 24 countries throughout Europe, adding value to healthcare organizations and assisting in the more cost-effective provision of high-quality services at home. As a result, this component spurs market expansion.
By Product
By Technology
By Application
By End Use
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Europe In Vitro Diagnostics Market
5.1. COVID-19 Landscape: Europe In Vitro Diagnostics Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Europe In Vitro Diagnostics Market, By Product
8.1. Europe In Vitro Diagnostics Market, by Product, 2024-2033
8.1.1. Instruments
8.1.1.1. Market Revenue and Forecast (2021-2033)
8.1.2. Reagents
8.1.2.1. Market Revenue and Forecast (2021-2033)
8.1.3. Services
8.1.3.1. Market Revenue and Forecast (2021-2033)
Chapter 9. Europe In Vitro Diagnostics Market, By Technology
9.1. Europe In Vitro Diagnostics Market, by Technology, 2024-2033
9.1.1. Immunology
9.1.1.1. Market Revenue and Forecast (2021-2033)
9.1.2. Haematology
9.1.2.1. Market Revenue and Forecast (2021-2033)
9.1.3. Clinical Chemistry
9.1.3.1. Market Revenue and Forecast (2021-2033)
9.1.4. Molecular Diagnostics
9.1.4.1. Market Revenue and Forecast (2021-2033)
9.1.5. Coagulation
9.1.5.1. Market Revenue and Forecast (2021-2033)
9.1.6. Microbiology
9.1.6.1. Market Revenue and Forecast (2021-2033)
9.1.7. Others
9.1.7.1. Market Revenue and Forecast (2021-2033)
Chapter 10. Europe In Vitro Diagnostics Market, By Application
10.1. Europe In Vitro Diagnostics Market, by Application, 2024-2033
10.1.1. Infectious Diseases
10.1.1.1. Market Revenue and Forecast (2021-2033)
10.1.2. Diabetes
10.1.2.1. Market Revenue and Forecast (2021-2033)
10.1.3. Oncology/Cancer
10.1.3.1. Market Revenue and Forecast (2021-2033)
10.1.4. Cardiology
10.1.4.1. Market Revenue and Forecast (2021-2033)
10.1.5. Nephrology
10.1.5.1. Market Revenue and Forecast (2021-2033)
10.1.6. Autoimmune Diseases
10.1.6.1. Market Revenue and Forecast (2021-2033)
10.1.7. Drug Testing
10.1.7.1. Market Revenue and Forecast (2021-2033)
10.1.8. Other
10.1.8.1. Market Revenue and Forecast (2021-2033)
Chapter 11. Europe In Vitro Diagnostics Market, By End Use
11.1. Europe In Vitro Diagnostics Market, by End Use, 2024-2033
11.1.1. Hospitals
11.1.1.1. Market Revenue and Forecast (2021-2033)
11.1.2. Laboratories
11.1.2.1. Market Revenue and Forecast (2021-2033)
11.1.3. Home Care
11.1.3.1. Market Revenue and Forecast (2021-2033)
11.1.4. Others
11.1.4.1. Market Revenue and Forecast (2021-2033)
Chapter 12. Europe In Vitro Diagnostics Market, Regional Estimates and Trend Forecast
12.1. Europe
12.1.1. Market Revenue and Forecast, by Product (2021-2033)
12.1.2. Market Revenue and Forecast, by Technology (2021-2033)
12.1.3. Market Revenue and Forecast, by Application (2021-2033)
12.1.4. Market Revenue and Forecast, by End Use (2021-2033)
Chapter 13. Company Profiles
13.1. Bio-Rad Laboratories, Inc
13.1.1. Company Overview
13.1.2. Product Offerings
13.1.3. Financial Performance
13.1.4. Recent Initiatives
13.2. Abbott
13.2.1. Company Overview
13.2.2. Product Offerings
13.2.3. Financial Performance
13.2.4. Recent Initiatives
13.3. Sysmex Corporation
13.3.1. Company Overview
13.3.2. Product Offerings
13.3.3. Financial Performance
13.3.4. Recent Initiatives
13.4. BD
13.4.1. Company Overview
13.4.2. Product Offerings
13.4.3. Financial Performance
13.4.4. Recent Initiatives
13.5. BIOMÉRIEUX
13.5.1. Company Overview
13.5.2. Product Offerings
13.5.3. Financial Performance
13.5.4. Recent Initiatives
13.6. Danaher
13.6.1. Company Overview
13.6.2. Product Offerings
13.6.3. Financial Performance
13.6.4. Recent Initiatives
13.7. F. Hoffmann-La Roche Ltd
13.7.1. Company Overview
13.7.2. Product Offerings
13.7.3. Financial Performance
13.7.4. Recent Initiatives
13.8. Siemens
13.8.1. Company Overview
13.8.2. Product Offerings
13.8.3. Financial Performance
13.8.4. Recent Initiatives
13.9. QIAGEN
13.9.1. Company Overview
13.9.2. Product Offerings
13.9.3. Financial Performance
13.9.4. Recent Initiatives
13.10. Thermo Fisher Scientific Inc
13.10.1. Company Overview
13.10.2. Product Offerings
13.10.3. Financial Performance
13.10.4. Recent Initiatives
Chapter 14. Research Methodology
14.1. Primary Research
14.2. Secondary Research
14.3. Assumptions
Chapter 15. Appendix
15.1. About Us
15.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others