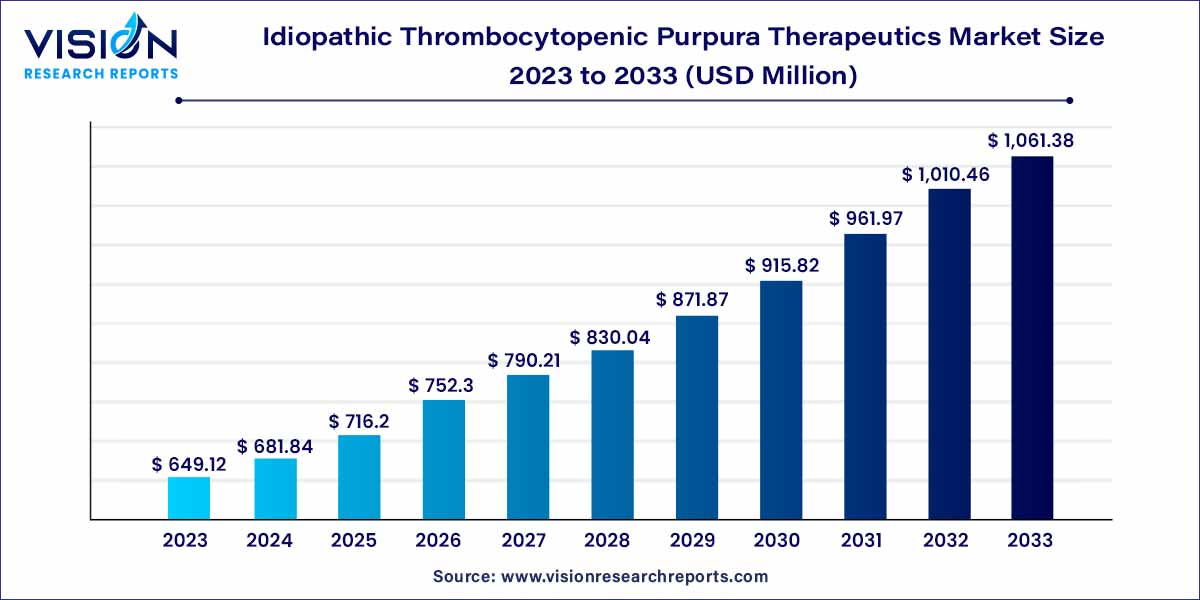
The global idiopathic thrombocytopenic purpura therapeutics market size was estimated at around USD 649.12 million in 2023 and it is projected to hit around USD 1,061.38 million by 2033, growing at a CAGR of 5.04% from 2024 to 2033.
Idiopathic thrombocytopenic purpura, commonly known as ITP, is a hematologic disorder characterized by a low platelet count, resulting in an increased risk of bleeding. The therapeutics market for ITP has witnessed significant developments in recent years, driven by advancements in research, innovation in treatment modalities, and a growing understanding of the condition.
The growth of the idiopathic thrombocytopenic purpura (ITP) therapeutics market is propelled by several key factors. Firstly, increased awareness and understanding of ITP among healthcare professionals and the general population have led to earlier diagnosis and intervention, fostering a greater demand for effective therapeutics. Additionally, ongoing research and development activities in the field have resulted in the emergence of novel treatment modalities, expanding the available options for managing this hematologic disorder. The collaborative efforts and strategic partnerships within the pharmaceutical industry have accelerated the development and commercialization of innovative ITP therapies, further contributing to market growth. Moreover, advancements in diagnostic technologies and a deeper comprehension of the underlying pathophysiology of ITP have facilitated the development of targeted and personalized treatment approaches, enhancing the overall efficacy of therapeutic interventions. As a result, the ITP therapeutics market is poised for sustained growth, driven by a combination of heightened awareness, research breakthroughs, and the continuous pursuit of improved patient outcomes.
| Report Coverage | Details |
| Market Revenue by 2033 | USD 1,061.38 million |
| Growth Rate from 2024 to 2033 | CAGR of 5.04% |
| Revenue Share of North America in 2023 | 40% |
| CAGR of Asia Pacific from 2024 to 2033 | 6.25% |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
Based on product, the corticosteroids segment dominated the market with the largest revenue share of 31% in 2023. Because they are usually employed as the first line of treatment for patients with ITP, corticosteroids have a high utilization rate. Additionally, these medicines' lower pricing and patients' generally positive responses have helped this market sector grow in utilization rates, particularly in developing and emerging markets.
The TPO-RA segment is expected to grow at a faster CAGR of 6.63% over the forecast period. The TPO-RA segment is the most promising. One of the main drivers of this market is the higher response rates seen in patients treated with Romiplostim and Eltrombopag. In individuals experiencing post-splenectomy relapses, TPO-RA is regarded as a feasible therapy option and is typically used when splenectomy fails.
Based on disease type, the market is segmented into acute ITP, chronic, and others. Acute ITP is a self-limiting form of the condition typically seen in children. It is characterized by a sudden onset of low platelet counts and resolves within six months in the majority of cases. The management of acute ITP often focuses on supportive care and close monitoring. Given its self-resolving nature, the market for acute ITP therapeutics may be relatively smaller compared to chronic ITP.
Chronic ITP refers to a persistent or recurrent condition that lasts more than six months. It can affect both children and adults. The management of chronic ITP aims to increase platelet counts and prevent bleeding episodes. Therapeutic options for chronic ITP include corticosteroids, thrombopoietin receptor agonists (TPO-RAs), immunosuppressants, and splenectomy (surgical spleen removal). The chronic ITP therapeutics market is likely to have a larger share than acute ITP due to the longer duration of treatment and a higher prevalence of chronic cases.
North America led the market with largest revenue share of 40% in 2023. The ITP therapeutics market's growth in North America was influenced by factors such as advancements in treatment options, increasing awareness and diagnosis of ITP, and the prevalence of the condition.
Asia Pacific is anticipated to grow at the fastest CAGR of 6.25% over the forecast period. This is attributed to several causes, including rising ITP therapeutic market penetration rates in emerging markets like South Korea, China, Taiwan, and India and the abundance of undiscovered business prospects in these nations. It is also anticipated that the availability of supportive government initiatives in Australia and Japan will fuel regional market expansion.
By Product
By Disease Type
By Region
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Idiopathic Thrombocytopenic Purpura Therapeutics Market
5.1. COVID-19 Landscape: Idiopathic Thrombocytopenic Purpura Therapeutics Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Idiopathic Thrombocytopenic Purpura Therapeutics Market, By Product
8.1. Idiopathic Thrombocytopenic Purpura Therapeutics Market, by Product, 2024-2033
8.1.1. Corticosteroids
8.1.1.1. Market Revenue and Forecast (2021-2033)
8.1.2. IVIG
8.1.2.1. Market Revenue and Forecast (2021-2033)
8.1.3. Anti-D Immunoglobulins
8.1.3.1. Market Revenue and Forecast (2021-2033)
8.1.4. TPO-RA
8.1.4.1. Market Revenue and Forecast (2021-2033)
8.1.5. Others
8.1.5.1. Market Revenue and Forecast (2021-2033)
Chapter 9. Global Idiopathic Thrombocytopenic Purpura Therapeutics Market, By Disease Type
9.1. Idiopathic Thrombocytopenic Purpura Therapeutics Market, by Disease Type, 2024-2033
9.1.1. Acute ITP
9.1.1.1. Market Revenue and Forecast (2021-2033)
9.1.2. Chronic
9.1.2.1. Market Revenue and Forecast (2021-2033)
9.1.3. Others
9.1.3.1. Market Revenue and Forecast (2021-2033)
Chapter 10. Global Idiopathic Thrombocytopenic Purpura Therapeutics Market, Regional Estimates and Trend Forecast
10.1. North America
10.1.1. Market Revenue and Forecast, by Product (2021-2033)
10.1.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.1.3. U.S.
10.1.3.1. Market Revenue and Forecast, by Product (2021-2033)
10.1.3.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.1.4. Rest of North America
10.1.4.1. Market Revenue and Forecast, by Product (2021-2033)
10.1.4.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.2. Europe
10.2.1. Market Revenue and Forecast, by Product (2021-2033)
10.2.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.2.3. UK
10.2.3.1. Market Revenue and Forecast, by Product (2021-2033)
10.2.3.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.2.4. Germany
10.2.4.1. Market Revenue and Forecast, by Product (2021-2033)
10.2.4.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.2.5. France
10.2.5.1. Market Revenue and Forecast, by Product (2021-2033)
10.2.5.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.2.6. Rest of Europe
10.2.6.1. Market Revenue and Forecast, by Product (2021-2033)
10.2.6.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.3. APAC
10.3.1. Market Revenue and Forecast, by Product (2021-2033)
10.3.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.3.3. India
10.3.3.1. Market Revenue and Forecast, by Product (2021-2033)
10.3.3.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.3.4. China
10.3.4.1. Market Revenue and Forecast, by Product (2021-2033)
10.3.4.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.3.5. Japan
10.3.5.1. Market Revenue and Forecast, by Product (2021-2033)
10.3.5.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.3.6. Rest of APAC
10.3.6.1. Market Revenue and Forecast, by Product (2021-2033)
10.3.6.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.4. MEA
10.4.1. Market Revenue and Forecast, by Product (2021-2033)
10.4.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.4.3. GCC
10.4.3.1. Market Revenue and Forecast, by Product (2021-2033)
10.4.3.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.4.4. North Africa
10.4.4.1. Market Revenue and Forecast, by Product (2021-2033)
10.4.4.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.4.5. South Africa
10.4.5.1. Market Revenue and Forecast, by Product (2021-2033)
10.4.5.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.4.6. Rest of MEA
10.4.6.1. Market Revenue and Forecast, by Product (2021-2033)
10.4.6.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.5. Latin America
10.5.1. Market Revenue and Forecast, by Product (2021-2033)
10.5.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.5.3. Brazil
10.5.3.1. Market Revenue and Forecast, by Product (2021-2033)
10.5.3.2. Market Revenue and Forecast, by Disease Type (2021-2033)
10.5.4. Rest of LATAM
10.5.4.1. Market Revenue and Forecast, by Product (2021-2033)
10.5.4.2. Market Revenue and Forecast, by Disease Type (2021-2033)
Chapter 11. Company Profiles
11.1. Amgen Inc.
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. F. Hoffmann-La Roche Ltd
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. Grifols, S.A.
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. GSK plc.
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. LTE Scientific
11.5. Shangxian Minimal Invassive Inc.
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. INTROMEDIC
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. Medtronic
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. FUJIFILM Holdings Corporation
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Olympus Corporation
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. JINSHAN Science & Technology (Group) Co., Ltd.
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others