The global influenza treatment market size was estimated at around USD 5.95 billion in 2023 and it is projected to hit around USD 6.71 billion by 2033, growing at a CAGR of 1.21% from 2024 to 2033.
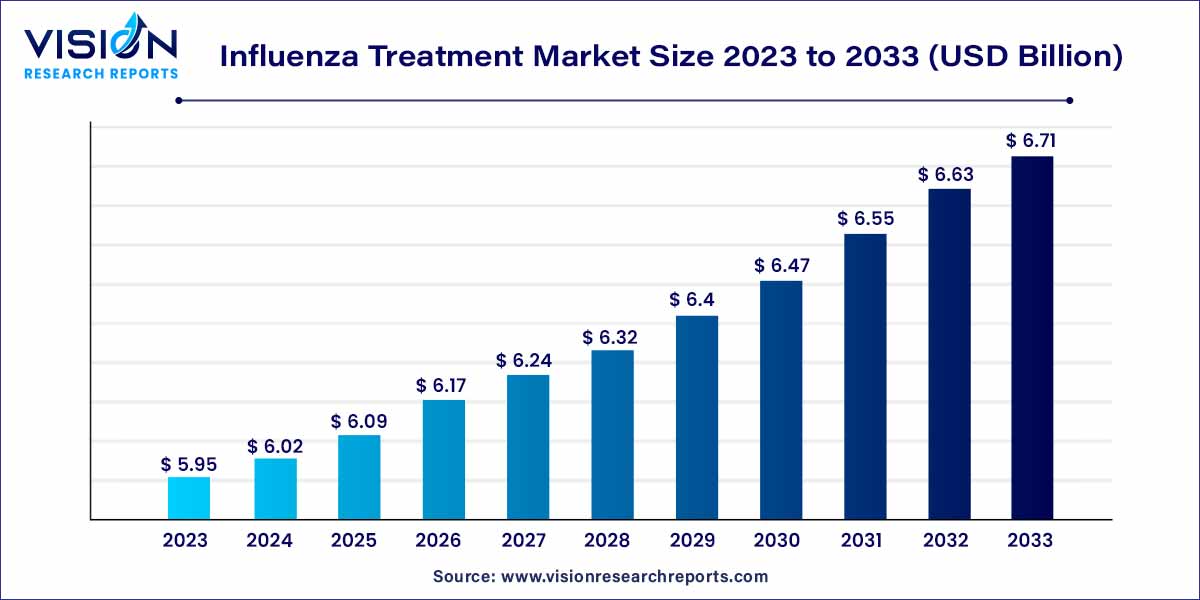
The influenza treatment market is a dynamic and critical segment within the pharmaceutical and healthcare industry. Influenza, commonly known as the flu, is a highly contagious respiratory illness caused by influenza viruses. As a result, the demand for effective treatments and therapeutics to manage and mitigate the impact of influenza remains a key focus area for pharmaceutical companies and healthcare providers.
The robust growth of the influenza treatment market can be attributed to several key factors. Firstly, ongoing advancements in medical research and technology contribute to the development of more effective antiviral medications and vaccines, enhancing the overall treatment landscape. Additionally, heightened awareness of influenza's potential severity, coupled with increasing initiatives for vaccination campaigns globally, fuels the demand for preventive measures. The ever-present threat of new influenza strains and the need for rapid response mechanisms stimulate continuous research and development, driving innovation within the market. Collaborations between pharmaceutical companies, government agencies, and research institutions play a pivotal role in accelerating treatment discoveries and ensuring widespread accessibility. Moreover, the market's resilience is fortified by the collective commitment to public health, resulting in a continuous evolution of strategies and products aimed at mitigating the impact of influenza. As these growth factors intertwine, the influenza treatment market is poised for sustained expansion in the foreseeable future.
| Report Coverage | Details |
| Market Revenue by 2033 | USD 6.71 billion |
| Growth Rate from 2024 to 2033 | CAGR of 1.22% |
| Revenue Share of North America in 2023 | 42% |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
The oseltamivir phosphate secured the largest market share 28% in 2023. Oseltamivir Phosphate, an antiviral drug approved for treating acute influenza in patients aged 2 weeks and older, is effective against Type A and B influenza. Its approval extends to both treatment and prevention within the community. Notably, in July 2020, Novadoz obtained FDA approval for a generic version of Tamiflu and introduced Tykosin. Tamiflu, available in various dosages (30 mg, 40 mg, and 75 mg), hinders influenza virus replication, reducing the severity and duration of flu symptoms when administered early in the illness. Consequently, these factors contribute significantly to the segment's growth.
Baloxavir marboxil is poised to exhibit the highest growth rate in the forecast period. This antiviral drug, approved by the U.S. FDA in 2018, is gaining traction for its oral administration in treating influenza. The increasing demand for baloxavir marboxil is expected to propel market growth, as it inhibits a distinct viral protein, the cap-dependent endonuclease, displaying efficacy against both influenza A and B viruses. Furthermore, its widespread adoption for influenza treatment has opened avenues for novel combination therapies, integrating medications with diverse mechanisms of action.
The oral route segment had the largest market share of 70% in 2023. This prevalence is attributed to the widespread use of the oral route for drug administration, owing to its convenience, non-invasiveness, and high patient compliance. According to the National Center for Biotechnology Information (NCBI), approximately 60% of established drug products are commercially administered orally, representing a majority share across various pharmaceutical formulations for human medication. The oral drug category continues to evolve, offering the potential for positive patient outcomes. Moreover, the availability of diverse drugs, including Peramivir, Zanamivir, Baloxavir Marboxil, and Oseltamivir Phosphate in oral forms, has further fueled the demand for this administration route.
Additionally, the other segment is anticipated to experience the fastest growth rate during the forecast period. This growth is driven by an increased demand for parenteral and intravenous routes of administration, which offer heightened safety and efficacy in flu medication. According to NCBI, Peramivir, for instance, presents a single-dose intravenous (IV) treatment option for influenza, commonly employed in treating patients with complicated and high-risk influenza cases. Consequently, these factors contribute to the robust growth of the other route of administration segment.
The hospital pharmacies segment captured the maximum market share of 48% in 2023. This dominance is primarily attributed to the escalating rates of hospitalizations and the growing demand for prescribed medications for treating influenza. Additionally, the majority of hospitals offer drugs at discounted prices, fostering widespread adoption of these medications. The proliferation of pharmacies within hospital and clinic settings has further propelled revenue growth, facilitated by an increased uptake of over-the-counter drugs. Consequently, this segment is poised to experience heightened demand for products throughout the forecast period.

Concurrently, the online pharmacies segment is projected to exhibit the fastest growth rate in the forecast period. This anticipation stems from the advantages of rapid access to medical products, privacy, cost efficiency, and the extensive availability of medicines, all from the comfort of one's home. Online pharmacies offer an efficient distribution channel, particularly beneficial for the elderly, disabled individuals, and those residing in remote locations, providing a convenient and swift avenue for obtaining necessary medications. Furthermore, the global pandemic has contributed to the increased adoption of online pharmacies due to restrictions on movement. The escalating internet penetration and the growing necessity for delivering medications to remote areas collectively propel the overall market growth.
North America dominated the market with the largest market share of 42% in 2023. The region's robust performance is driven by factors such as an expanding population susceptible to respiratory illnesses, the continual rise of seasonal flu, increased hospitalization rates due to such conditions, and the presence of well-established healthcare facilities. The elevated risk of seasonal flu, especially among vulnerable populations like small children, the elderly, pregnant women, and healthcare workers, has propelled the demand for influenza treatment in the region. Seasonal flu, a prevalent health concern occurring typically between December and February, underscores the necessity for effective therapeutics. Key market players are strategically focusing on developing innovative therapeutics to address the growing prevalence of influenza. For instance, in August 2022, Genentech obtained supplemental New Drug Application approval for Xofluza, a treatment for uncomplicated acute influenza.

In contrast, Asia Pacific is poised to exhibit the fastest growth over the forecast period. This growth is attributed to the increasing elderly population in Asian countries, prone to respiratory conditions and supported by enhanced influenza surveillance practices in the region. Additionally, factors such as developing healthcare infrastructure, a robust presence of pharmaceutical companies, rising demand for influenza drugs, and improved control strategies collectively contribute to this growth trajectory. Regional players are actively introducing novel treatment options into the market. For example, in March 2023, TaiGen Biotechnology Co., Ltd. entered into a licensing agreement with Joincare Pharmaceutical Group Industry Co., Ltd. for the development and commercialization of TG-1000 in China, targeting infections caused by influenza-B, influenza-A, avian flu H7N9, and Tamiflu-resistant viruses.
By Treatment
By Route of Administration
By Distribution Channel
By Region
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Treatment Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Influenza Treatment Market
5.1. COVID-19 Landscape: Influenza Treatment Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Influenza Treatment Market, By Treatment
8.1. Influenza Treatment Market, by Treatment, 2024-2033
8.1.1 Peramivir
8.1.1.1. Market Revenue and Forecast (2021-2033)
8.1.2. Zanamivir
8.1.2.1. Market Revenue and Forecast (2021-2033)
8.1.3. Baloxavir Marboxil
8.1.3.1. Market Revenue and Forecast (2021-2033)
8.1.4. Oseltamivir Phosphate
8.1.4.1. Market Revenue and Forecast (2021-2033)
8.1.5. Others
8.1.5.1. Market Revenue and Forecast (2021-2033)
Chapter 9. Global Influenza Treatment Market, By Route of Administration
9.1. Influenza Treatment Market, by Route of Administration, 2024-2033
9.1.1. Oral
9.1.1.1. Market Revenue and Forecast (2021-2033)
9.1.2. Others
9.1.2.1. Market Revenue and Forecast (2021-2033)
Chapter 10. Global Influenza Treatment Market, By Distribution Channel
10.1. Influenza Treatment Market, by Distribution Channel, 2024-2033
10.1.1. Hospital Pharmacies
10.1.1.1. Market Revenue and Forecast (2021-2033)
10.1.2. Retail Pharmacies
10.1.2.1. Market Revenue and Forecast (2021-2033)
10.1.3. Online Pharmacies
10.1.3.1. Market Revenue and Forecast (2021-2033)
Chapter 11. Global Influenza Treatment Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.1.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.1.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.1.4.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.1.4.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.1.5.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.1.5.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.2.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.2.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.2.4.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.2.4.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.2.5.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.2.5.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.2.6.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.2.6.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.2.7.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.2.7.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.3.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.3.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.3.4.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.3.4.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.3.5.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.3.5.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.3.6.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.3.6.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.3.7.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.3.7.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.4.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.4.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.4.4.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.4.4.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.4.5.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.4.5.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.4.6.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.4.6.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.4.7.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.4.7.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.5.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.5.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.5.4.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.5.4.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Treatment (2021-2033)
11.5.5.2. Market Revenue and Forecast, by Route of Administration (2021-2033)
11.5.5.3. Market Revenue and Forecast, by Distribution Channel (2021-2033)
Chapter 12. Company Profiles
12.1. NATCO Pharma Limited.
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Novartis AG
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. F. Hoffmann-La Roche Ltd
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. BioCryst Pharmaceuticals, Inc.
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Sanofi
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. GSK plc.
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Viatris Inc. (MYLAN)
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Teva Pharmaceutical Industries Ltd.
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Daiichi Sankyo Company, Limited.
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. AstraZeneca
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others