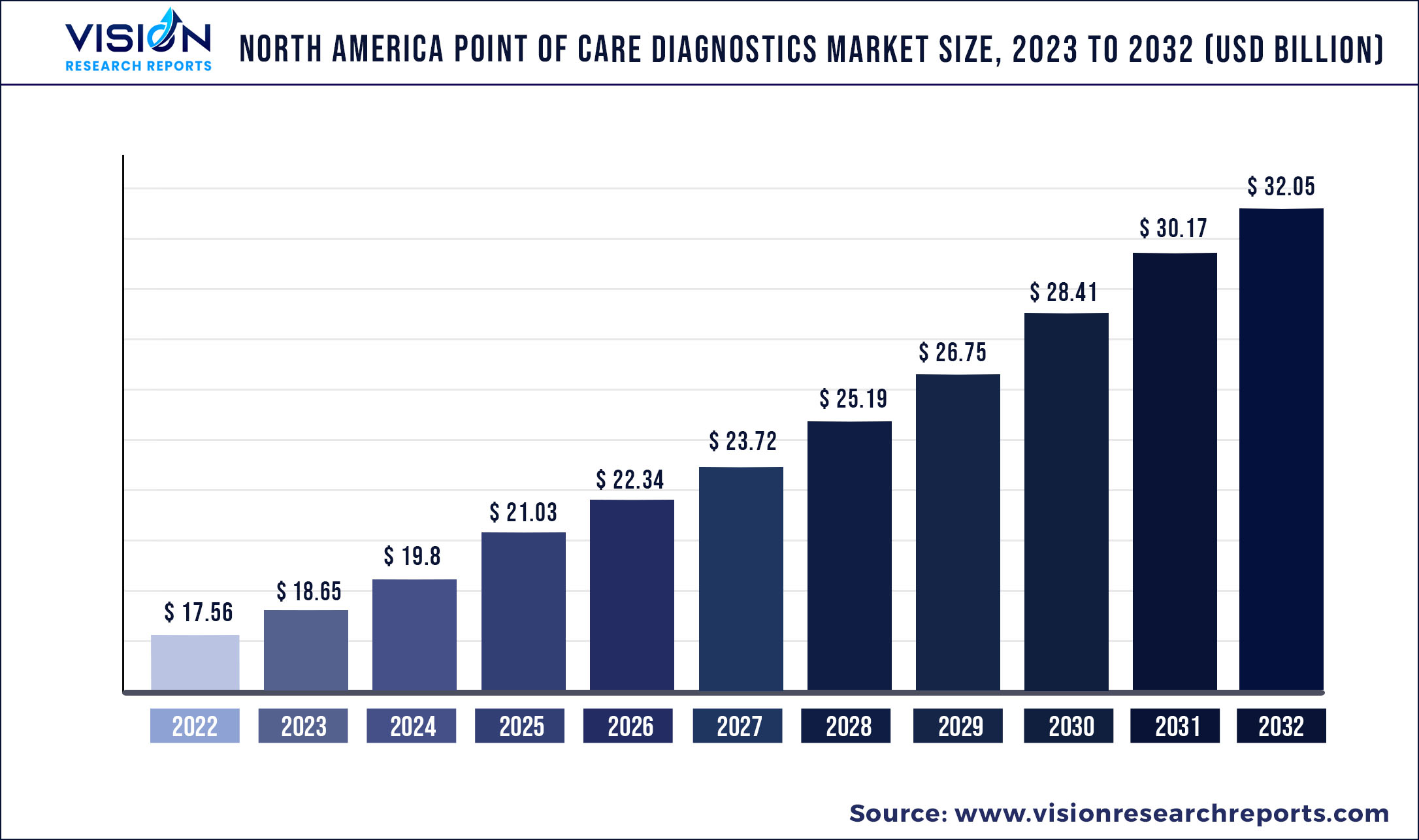
The North America point of care diagnostics market size was estimated at around USD 17.56 billion in 2022 and it is projected to hit around USD 32.05 billion by 2032, growing at a CAGR of 6.2% from 2023 to 2032.
Key Pointers
| Report Coverage | Details |
| Market Size in 2022 | USD 17.56 billion |
| Revenue Forecast by 2032 | USD 32.05 billion |
| Growth rate from 2023 to 2032 | CAGR of 6.2% |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Companies Covered | F. Hoffmann-La Roche AG; QIAGEN; Danaher Corporation; BD; bioMérieux SA; Abbott; Siemens Healthineers A.G.; Zoetis, Inc.; Instrumentation Laboratory; Nova Biomedical; Trividia Health, Inc.; Quidel Corporation; Trinity Biotech; Sekisui Diagnostics; OraSure Technologies, Inc.; NIPRO, Spectral Medical, Inc. |
Point-of-care (POC) diagnosis refers to performing diagnostic tests at the site of the patient. The growing geriatric population and the ability of POC diagnostic tests to deliver immediate results are likely to fuel the demand for POC diagnostic tests. Furthermore, a rise in funding from the government & private institutions is a key factor driving the market’s growth. An increase in the adoption of mobile diagnostic devices in the region is also one of the key factors driving demand for POC testing.
The COVID-19 outbreak has positively impacted the market. This can primarily be attributed to the urgent need for manufacturing and introduction of rapid & portable diagnostic tests, systems, and accessories in the market that can presently assist in scaling up of COVID-19 testing. Moreover, the advent of rapid data-sharing platforms and solutions is expected to contribute to the development of COVID-19 point-of-care technologies, supplementing the market growth. Companies are actively working toward the development of rapid testing solutions. For instance, in November 2022, Sense Biodetection entered into a collaboration with Bio Nuclear Diagnostics to distribute Sense’s Veros POC, an instrument-free molecular COVID-19 test, in Canada.
Furthermore, increased funding from NIH, private foundations such as the Bill & Melinda Gates Foundation, and the U.S. department of defense (DOD), is anticipated to fuel the market growth. For instance, in November 2021, Hyperfine, Inc., announced receiving USD 3.3 million in funding from the Bill & Melinda Gates Foundation to broaden the usage of portable MRI technology in various countries. Similarly, in August 2022, Nanopath, Inc. received funding of USD 10 million from Medtech Convergence Fund and Norwest Venture Partners with participation from Green D Ventures and Gingerbread Capital. The funding would help develop point-of-care diagnostics for women’s health.
In the U.S., the population aged 65 and older is estimated to double from 52 million in 2018 to 95 million by 2060, which is likely to increase demand for POC diagnostics. According to projections, there will be a 23% increase in the overall population. Aging increases the risk of diseases such as cardiovascular diseases and cancer. Therefore, an increase in the geriatric population is expected to boost the demand for continuous monitoring via facilities requiring point-of-care diagnostics, such as home healthcare and assisted living healthcare facilities.
Country Insights
The U.S. dominated the North America point-of-care market in 2022 due to the presence of key players such as Abbott, BIOMERIEUX, BD, Siemens Healthineers AG, QIAGEN, Quidel Corporation, and Quest Diagnostics is positively influencing the market growth.
Canada is estimated to witness the fastest growth rate over the coming years. Usage of POC diagnostic devices in these regions is increasing owing to the increasing prevalence of target diseases such as cardio-metabolic disorders, infectious diseases, and increasing cases of drug abuse. Increasing demand for rapid and early diagnosis leading to better treatment alternatives and presence of health-conscious population are also considered as other factors fueling industrial growth.
North America Point Of Care Diagnostics Market Segmentations:
By Product
By End-use
By Type
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on North America Point Of Care Diagnostics Market
5.1. COVID-19 Landscape: North America Point Of Care Diagnostics Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. North America Point Of Care Diagnostics Market, By Product
8.1. North America Point Of Care Diagnostics Market, by Product, 2023-2032
8.1.1 Glucose Testing
8.1.1.1. Market Revenue and Forecast (2020-2032)
8.1.2. HbA1c Testing
8.1.2.1. Market Revenue and Forecast (2020-2032)
8.1.3. Coagulation
8.1.3.1. Market Revenue and Forecast (2020-2032)
8.1.4. Fertility/Pregnancy
8.1.4.1. Market Revenue and Forecast (2020-2032)
8.1.5. Infectious Diseases
8.1.5.1. Market Revenue and Forecast (2020-2032)
8.1.6. Cardiac Markers
8.1.6.1. Market Revenue and Forecast (2020-2032)
8.1.7. Thyroid Stimulating Hormone
8.1.7.1. Market Revenue and Forecast (2020-2032)
8.1.8. Primary Care Systems
8.1.8.1. Market Revenue and Forecast (2020-2032)
8.1.9. Feces
8.1.9.1. Market Revenue and Forecast (2020-2032)
8.1.10. Lipid Testing
8.1.10.1. Market Revenue and Forecast (2020-2032)
8.1.11. Cancer Marker
8.1.11.1. Market Revenue and Forecast (2020-2032)
8.1.12. Blood Gas/Electrolytes
8.1.12.1. Market Revenue and Forecast (2020-2032)
8.1.13. Ambulatory Chemistry
8.1.13.1. Market Revenue and Forecast (2020-2032)
8.1.14. Drug Abuse Testing
8.1.14.1. Market Revenue and Forecast (2020-2032)
8.1.15. Urinalysis/Nephrology
8.1.15.1. Market Revenue and Forecast (2020-2032)
Chapter 9. North America Point Of Care Diagnostics Market, By End-use
9.1. North America Point Of Care Diagnostics Market, by End-use, 2023-2032
9.1.1. Hospitals
9.1.1.1. Market Revenue and Forecast (2020-2032)
9.1.2. Laboratories
9.1.2.1. Market Revenue and Forecast (2020-2032)
9.1.3. Home Care
9.1.3.1. Market Revenue and Forecast (2020-2032)
9.1.4. Clinic
9.1.4.1. Market Revenue and Forecast (2020-2032)
Chapter 10. North America Point Of Care Diagnostics Market, By Type
10.1. North America Point Of Care Diagnostics Market, by Type, 2023-2032
10.1.1. LDTs
10.1.1.1. Market Revenue and Forecast (2020-2032)
10.1.2. Others
10.1.2.1. Market Revenue and Forecast (2020-2032)
Chapter 11. North America Point Of Care Diagnostics Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Product (2020-2032)
11.1.2. Market Revenue and Forecast, by End-use (2020-2032)
11.1.3. Market Revenue and Forecast, by Type (2020-2032)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Product (2020-2032)
11.1.4.2. Market Revenue and Forecast, by End-use (2020-2032)
11.1.4.3. Market Revenue and Forecast, by Type (2020-2032)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Product (2020-2032)
11.1.5.2. Market Revenue and Forecast, by End-use (2020-2032)
11.1.5.3. Market Revenue and Forecast, by Type (2020-2032)
Chapter 12. Company Profiles
12.1. F. Hoffmann-La Roche AG
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. QIAGEN
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Danaher Corporation
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. BD
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. bioMérieux SA
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Siemens Healthineers A.G.
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Zoetis, Inc.
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Instrumentation Laboratory
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Nova Biomedical
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. Trividia Health, Inc.
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others