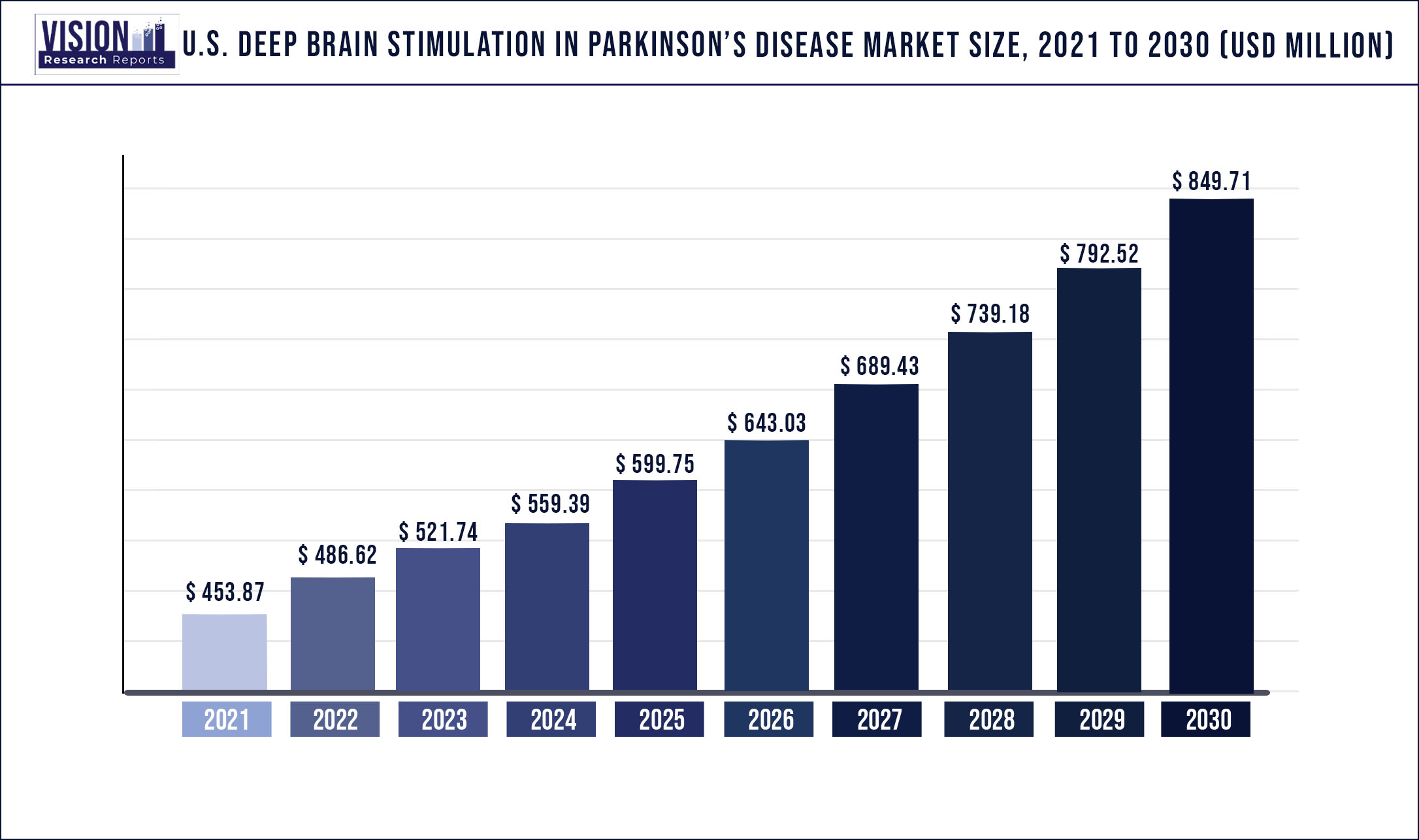
The U.S. deep brain stimulation in Parkinson’s disease market was estimated at USD 453.87 million in 2021 and it is expected to surpass around USD 849.71 million by 2030, poised to grow at a CAGR of 7.22% from 2022 to 2030.
Report Highlights
The rising prevalence of neurological conditions, such as Parkinson’s Disease (PD), increasing awareness, positive research outcomes, and growing investments in the development of transcranial stimulators are among the major factors driving the overall U.S DBS devices market.
DBS devices have been observed to be greatly effective in controlling the tremors associated with PD. They provide electrical stimulation to the basal ganglia, which results in suppressed neuronal activity that is generally spontaneous in patients suffering from PD. Low dopamine levels and other genetic factors are the main causes of PD. According to the Parkinson’s Foundation, about 1 million people are expected to be living with PD in the U.S. by 2020. Men are more susceptible to this disease than women.
Increasing investments for conducting R&D on PD have helped in raising awareness and boosting the development of new and innovative products and therapies for the treatment of this disease. For instance, the National Parkinson Foundation (NPF) provides funds to researchers and scientists with an aim to make advancements in this field. Extensive research activities for the inclusion and approval of deep brain stimulators in new application areas, such as full-body MRI scans and depression, are anticipated to boost the market growth over the forecast period. The DBS procedure price in the U.S. is around USD 96,000. It is expensive as compared with the cost of DBS procedures in countries such as the U.K. and Japan.
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 453.87 million |
| Revenue Forecast by 2030 | USD 849.71 million |
| Growth rate from 2022 to 2030 | CAGR of 7.22% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Product |
| Companies Covered | Boston Scientific Corporation; Abbott; Medtronic; Functional Neuromodulation; Nuvectra Corporation; Aleva Neurotherapeutics SA |
Product Insights
The dual-channel led the market and accounted for more than 55.0% share in 2021. High-frequency DBS has become a widely utilized procedure for the management of severe momentum disorders when the symptoms can no longer be improved by medical treatment. The procedure is safe, bilateral, and reversible. It can be performed by bilateral implantation of leads into the target areas. Growing adoption of double-channel DBS for the treatment of numerous neurological disorders, such as Parkinson’s disease, dystonia, Alzheimer’s disease, and epilepsy, is a key factor fueling segment growth.
Moreover, technological advancements and new product launches are further impelling segment growth. For instance, in January 2020, Abbott’s Infinity DBS system secured FDA approval for the treatment of Parkinson's disease from the U.S. FDA. This system will allow targeting of a specific area of the brain called internal Globus Pallidus (GPi), which is associated with Parkinson's disease symptoms. In addition, Vercise, Vercise PC, & Vercise Gevia DBS systems by Boston Scientific; Activa PC & Activa RC by Medtronic; and Infinity by Abbott are some of the key offerings under the segment.
The single-channel segment is anticipated to witness the fastest growth over the forecast period. A Deep Brain Stimulator (DBS) device is also known as brain pacemaker and has been clinically used over the past 25 years for the treatment of Parkinson's disease. The single-channel DBS systems are used for patients who have only one lead implanted. Healthcare professionals consider that a single channel offers the neurologist more programming options and provides better motor results, which led to the increased preference for single-channel DBS.
A rise in the geriatric population, which is more prone to developing Parkinson’s disease, and growing awareness about neurological movement disorders among patients are anticipated to impel the growth of the segment. For instance, as per the World Ageing 2019 report, it is estimated that globally, there were about 703 million people aged 65 years in 2019. Furthermore, in March 2018, the Brain & Spine Foundation (BSF) and Neurological Alliance launched “Brain Awareness Week,” a global campaign to increase awareness about progress in diagnosis, treatment, and prevention of neurological conditions. One of the key products offered under this segment is Activa SC, which is a single-channel DBS offered by Medtronic. Activa SC controls one-DBS electrode, which has been approved for the treatment of Parkinson’s disease.
Key Players
Market Segmentation
Product Outlook
Chapter 1.Introduction
1.1.Research Objective
1.2.Scope of the Study
1.3.Definition
Chapter 2.Research Methodology
2.1.Research Approach
2.2.Data Sources
2.3.Assumptions & Limitations
Chapter 3.Executive Summary
3.1.Market Snapshot
Chapter 4.Market Variables and Scope
4.1.Introduction
4.2.Market Classification and Scope
4.3.Industry Value Chain Analysis
4.3.1.Raw Material Procurement Analysis
4.3.2.Sales and Distribution Channel Analysis
4.3.3.Downstream Buyer Analysis
Chapter 5.COVID 19 Impact on U.S. Deep Brain Stimulation In Parkinson’s Disease Market
5.1.COVID-19 Landscape: U.S. Deep Brain Stimulation In Parkinson’s Disease Industry Impact
5.2.COVID 19 - Impact Assessment for the Industry
5.3.COVID 19 Impact: Global Major Government Policy
5.4.Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6.Market Dynamics Analysis and Trends
6.1.Market Dynamics
6.1.1.Market Drivers
6.1.2.Market Restraints
6.1.3.Market Opportunities
6.2.Porter’s Five Forces Analysis
6.2.1.Bargaining power of suppliers
6.2.2.Bargaining power of buyers
6.2.3.Threat of substitute
6.2.4.Threat of new entrants
6.2.5.Degree of competition
Chapter 7.Competitive Landscape
7.1.1.Company Market Share/Positioning Analysis
7.1.2.Key Strategies Adopted by Players
7.1.3.Vendor Landscape
7.1.3.1.List of Suppliers
7.1.3.2.List of Buyers
Chapter 8.Global U.S. Deep Brain Stimulation In Parkinson’s Disease Market, By Product
8.1.U.S. Deep Brain Stimulation In Parkinson’s Disease Market, by Product Type, 2020-2027
8.1.1.Single-channel
8.1.1.1.Market Revenue and Forecast (2016-2027)
8.1.2.Dual-channel
8.1.2.1.Market Revenue and Forecast (2016-2027)
Chapter 9.Global U.S. Deep Brain Stimulation In Parkinson’s Disease Market, Regional Estimates and Trend Forecast
9.1.U.S.
9.1.1.Market Revenue and Forecast, by Product (2016-2027)
Chapter 10.Company Profiles
10.1.Boston Scientific Corporation
10.1.1.Company Overview
10.1.2.Product Offerings
10.1.3.Financial Performance
10.1.4.Recent Initiatives
10.2.Abbott
10.2.1.Company Overview
10.2.2.Product Offerings
10.2.3.Financial Performance
10.2.4.Recent Initiatives
10.3.Medtronic
10.3.1.Company Overview
10.3.2.Product Offerings
10.3.3.Financial Performance
10.3.4.Recent Initiatives
10.4.Functional Neuromodulation
10.4.1.Company Overview
10.4.2.Product Offerings
10.4.3.Financial Performance
10.4.4.Recent Initiatives
10.5.Nuvectra Corporation
10.5.1.Company Overview
10.5.2.Product Offerings
10.5.3.Financial Performance
10.5.4.Recent Initiatives
10.6.Aleva Neurotherapeutics SA
10.6.1.Company Overview
10.6.2.Product Offerings
10.6.3.Financial Performance
10.6.4.Recent Initiatives
Chapter 11.Research Methodology
11.1.Primary Research
11.2.Secondary Research
11.3.Assumptions
Chapter 12.Appendix
12.1.About Us
12.2.Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others